Our Research
The immune system mounts destructive responses to protect the host from diverse threats. However, a trade-off emerges: if immune responses cause too much damage, they can compromise host tissue function. Conversely, if they fail to generate sufficient damage, the host may succumb to a given threat. How is the optimal balance achieved? The Wong lab investigates how groups of cells assemble, communicate, and compute to dynamically control immune responses in tissues. Many of our ongoing projects focus on T cells as a paradigm because subtle shifts in their control can lead to widely divergent host outcomes, including the successful elimination of threats, the induction of tolerance against foreign entities (e..g, commensal organisms), and the initiation of autoimmune disorders.
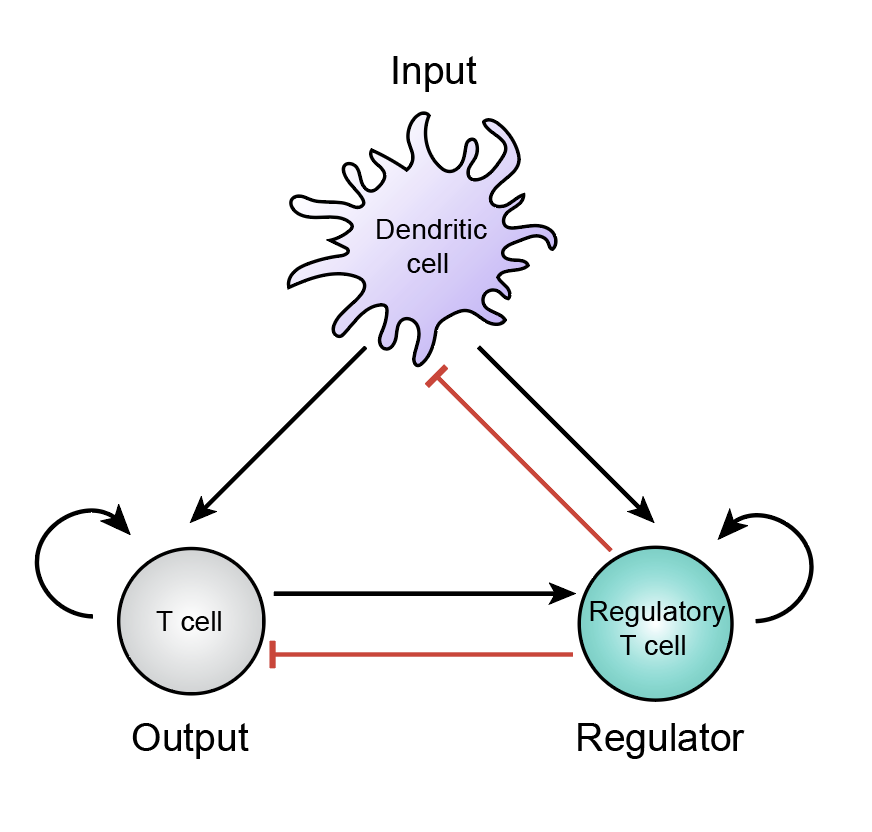
T cell circuit design principles
T cells communicate with a range of cell types through physical interactions and diffusible signals. This communication gives rise to different intercellular circuits—including feedback and feedforward—that control the magnitude of T cell responses over time and space. We are interested in defining minimal circuit modules across tissues and understanding their regulatory properties in various immunological settings, including autoimmunity, cancer, pregnancy, and infection. We are also interested in quantifying the precise margin of error in these circuits before T cell control begins to breakdown and initiates disease states.
Tissues vary widely in their characteristics—including functions, selection pressures, and regenerative capabilities—likely differing in terms of how much damage they can tolerate during immune responses before their basic physiology breaks down. How does the immune system account for these tissue-specific trade-offs? To answer this question, we investigate how immune cells migrate, organize, and interface with parenchyma throughout different organs. We also study mechanisms of tissue compartmentalization, which can restrict specific immune reactions to particular locations in space. A better appreciation of these organizational principles should aid in understanding why some tissues are more susceptible to certain disease states than others and engineering immunotherapies with tissue-specific efficacy.
Spatial assembly of the immune system across tissues
Spatial control of cytokine fields
Upon sensing threats, cells secrete numerous cytokines that diffuse and form gradients in three-dimensional space. The resulting cytokine fields facilitate communication and have multiple functions, including promoting localized host defence, protecting surrounding tissue from collateral damage, and fostering tissue repair. We investigate questions related to the control of cytokine diffusion during immune responses. For instance, how are functionally-opposing cytokine fields controlled to ensure that immune, parenchymal, and stromal cells adopt appropriate phenotypes at the correct coordinates in space? Moreover, how do cells interpret superimposed cytokine fields? Lastly, how do sustained breakdowns in the control of cytokine diffusion induce pathological phenotypes, such as fibrosis or tumorigenesis?
Our Approach
We combine the tools of immunology with interdisciplinary methods—including high-resolution fluorescence microscopy, computational approaches, and gene manipulations—to resolve, model, and perturb the control of immune responses in intact tissues. We also develop new imaging technologies and image analysis tools to better quantify the spatiotemporal dynamics of the immune system.